Electron microprobe analysis provides precise quantitative chemical analysis of elements in microarea of solid specimens, JEOL SuperProbe 8230 is equipped with:
5 wavelength X-Ray spectrometers (WDS) with 12 diffracting crystals (LiF, LiFL, LiFH, TAP, TAPH, PETL, PETH, PETJ, LDE1, LDE2, LDE3)
Energy-dispersive X-ray spectrometer (EDS)
Reflected and transmitted light microscope
Cathodoluminescence detector
Accessory: QUORUM Q150TE turbo-pumped carbon coater

Capabilities include
Full quantitative analysis. Detectable elements (from Be to U, excluding noble gases) are measured in a "spot" of <1 to 5 µm. Detection limits for most elements are down to 100 ppm or lower, depending on the element and settings.
Rapid qualitative analysis and phase identification in EDS mode.
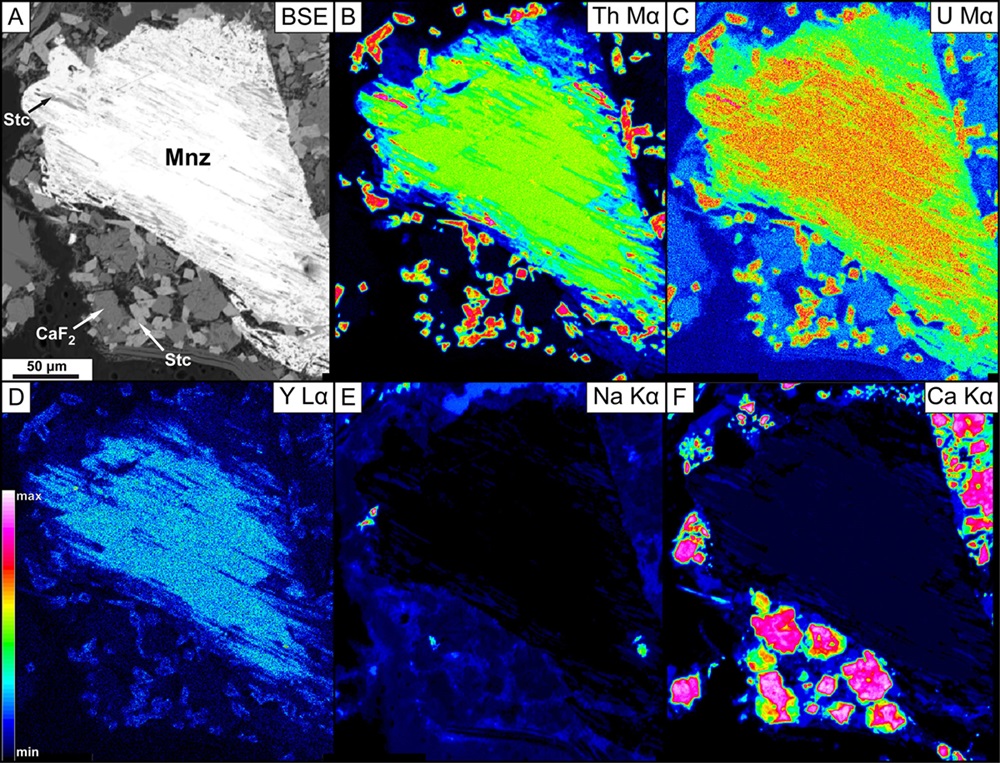
High-resolution quantitative chemical mapping of specimens on scales from below 100 nm to ca X cm
Imaging specimens using backscattered electron (BSE) and secondary electron (SE) signal
Cathodoluminescence (CL) imaging, CL images (grayscale or fake colors) can be acquired separately or synchronously with the WDS mapping.
Access
The EPMA facility is open to users from all departments of AGH University, as well as external reseach collaborators and industry partners. New users are encouraged to contact Gabriela Kozub-Budzyń (lato@agh.edu.pl) to discuss their projects and arrange for instrument time needed, pricing and booking.
The charge of the EPMA use is needed for the maintenance of the instrument, fee depend on the type of user and time used during workday or overnight.
Order form
Samples
The sample must be solid, stable under high vacuum and with top surface flat and polished. Before analysis they are coated with a thin film of electrically-conductive material (typically carbon; less comonly noble metals). Regular samples are polished round blocks/mounts/plates or thin sections of inorganic solids (e.g. rocks, heavy mineral concentrates, synthetic material, glasses, bones, fossils, etc.).
Optimal sample sizes:
round sample mounts 25.4 mm (1.0 inch) in diameter and up to 1.5 cm in height
thin sections, approximatley with dimensions of 46 x 27 x 1.5 mm
Available sample holders:
About the method
The principle of the EPMA method is very similar to the principle of Scanning Electron Microscope (SEM) method when it comes to generate X-rays and images. A solid sample placed in vacuum is bombarded with a focused beam of electrons (accelerating voltage 5 – 30 keV). This results in a multitude interactions between the beam electrons and atoms comprising the sample, and as it happens in a very small volume (<1 to 5 µm in diameter), it allows for “in situ” quantitative analysis.
The most important of these interactions is when a beam electron collides with an atom in the sample and causes an inner shell electron to be ejected from the atom. The vacancy leaves the atom in a high-energy excited state and this excess energy is lost by “jumping” of an outer shell electron to fill the inner shell vacancy, accompanied by the release of a photon. The frequency of this photon emission has a specific wavelength, which is characteristic for an element, and it is the range of X-rays.
The emitted photon has energy equal to the difference in energy between the two shells involved in the transition. For a given transition, each element will produce x-rays with a characteristic energy and wavelength which the electron microprobe uses to identify and quantify the elements present in the sample.
The characteristic X-rays of the element from an unknown sample are detected and counted by wavelength dispersive (WDS) spectrometers and the final concentration of the element in the sample is calculated based on the proportionality between the X-rays intensity in the unknown sample and the X-rays intensity produced by the known standard. As the crystal of a WDS spectrometer can only collect x-ray counts for one wavelength at a time, the analysis of multiple elements on a single spectrometer must be done sequentially.
An Energy Dispersive Spectrometry (EDS) detector can identify and discriminate the emitted x-rays by their energy, as well as it can count the X-rays of different energy, allowing semi-quantitative analysis.